Full text loading...
This review aims to illustrate the potential of kinetic analysis in general and microkinetic modeling in particular for rational catalyst design. Both ab initio calculations and experiments providing intrinsic kinetic data allow us to assess the effects of catalytic properties and reaction conditions on the activity and selectivity of the targeted reactions. Three complementary approaches for kinetic analysis of complex reaction networks are illustrated, using select examples of acid zeolite–catalyzed reactions from the authors' recent work. Challenges for future research aimed at defining targets for synthesis strategies that enable us to tune zeolite properties are identified.

Article metrics loading...

Full text loading...
Literature Cited


Data & Media loading...
Supplemental Material
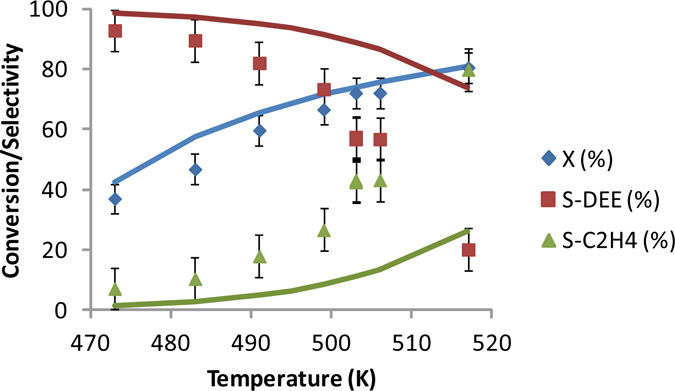
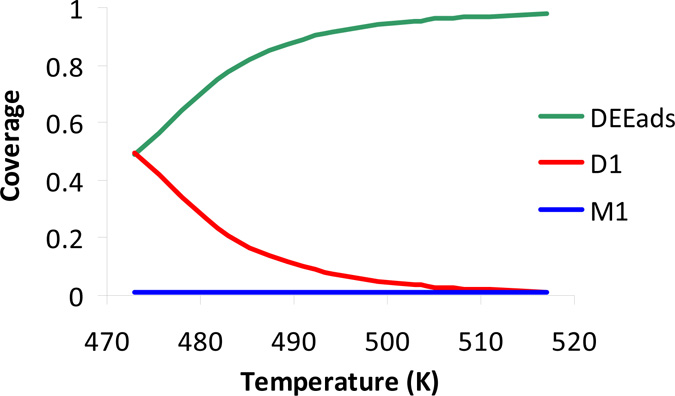
Download Supplemental Material (PDF). Includes Supplemental Tables 1-3, and Supplemental Figures 1-2 (also reproduced below). Supplemental Figure 1. Ethanol conversion (X), ethylene (S-C2H4) and diethyl ether (S-DEE) selectivity as a function of temperature at space time Wcat/FEtOH,0 = 6.5 kg smol-1 and PEtOH,0 = 24 kPa over H-ZSM-5. Symbols: experimental data are indicated with their 95% confidence interval. Full lines: model simulations obtained using the ab initio calculated rate coefficients (see text) Supplemental Figure 2. Simulated coverage of the most important surface intermediates, M1, D1 and DEE* as a function of temperature at space time Wcat/FEtOH,0 = 6.5 kg smol-1 and PEtOH,0 = 24 kPa over H-ZSM-5. Full lines: model simulations obtained using the ab initio calculated rate coefficients (see text).