Full text loading...
Resistance to the soybean cyst nematode (SCN) is a topic incorporating multiple mechanisms and multiple types of science. It is also a topic of substantial agricultural importance, as SCN is estimated to cause more yield damage than any other pathogen of soybean, one of the world's main food crops. Both soybean and SCN have experienced jumps in experimental tractability in the past decade, and significant advances have been made. The rhg1-b locus, deployed on millions of farm acres, has been durable and will remain important, but local SCN populations are gradually evolving to overcome rhg1-b. Multiple other SCN resistance quantitative trait loci (QTL) of proven value are now in play with soybean breeders. QTL causal gene discovery and mechanistic insights into SCN resistance are contributing to both basic and applied disciplines. Additional understanding of SCN and other cyst nematodes will also grow in importance and lead to novel disease control strategies.

Article metrics loading...

Full text loading...
Literature Cited


Data & Media loading...
Supplemental Material
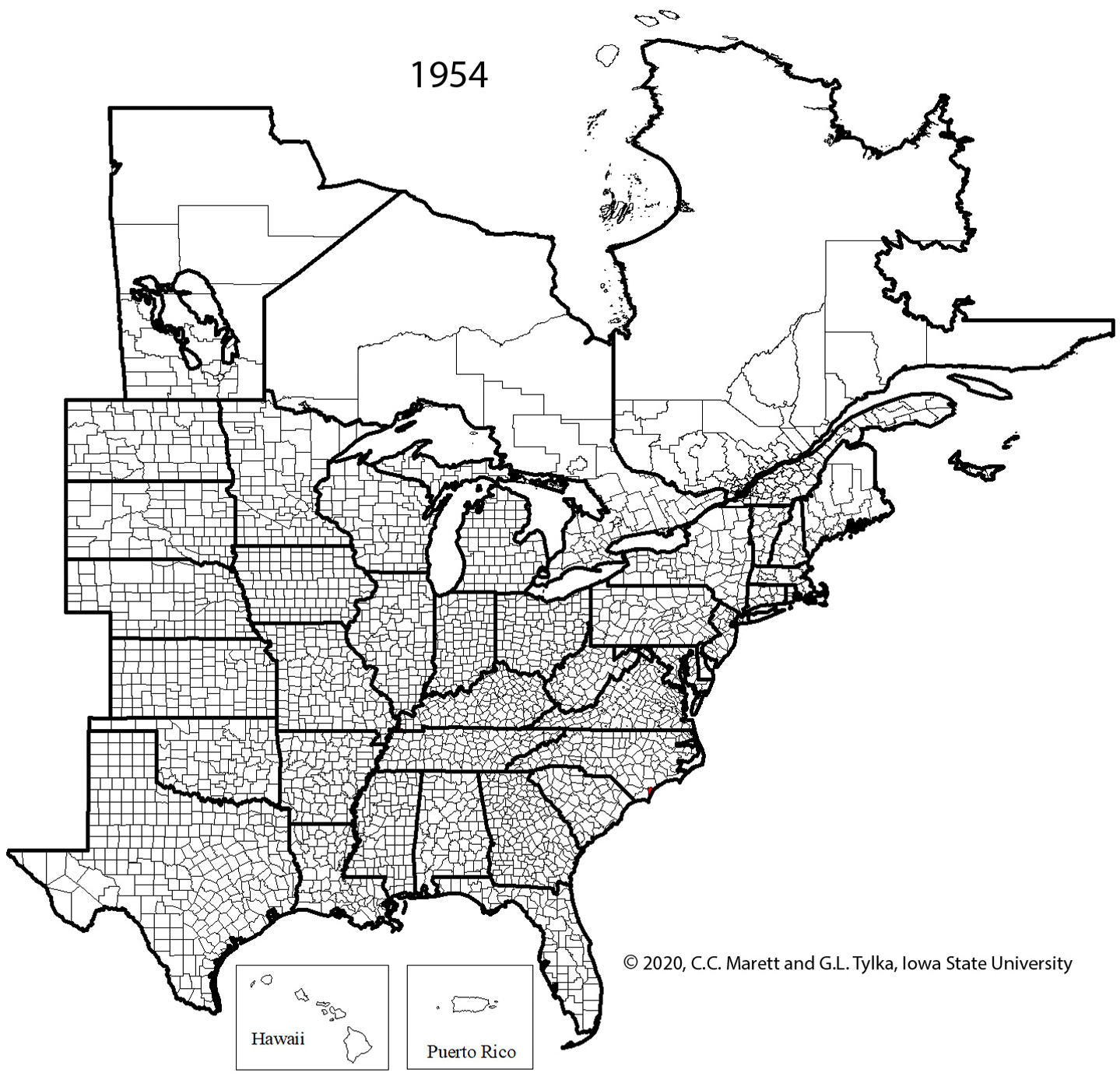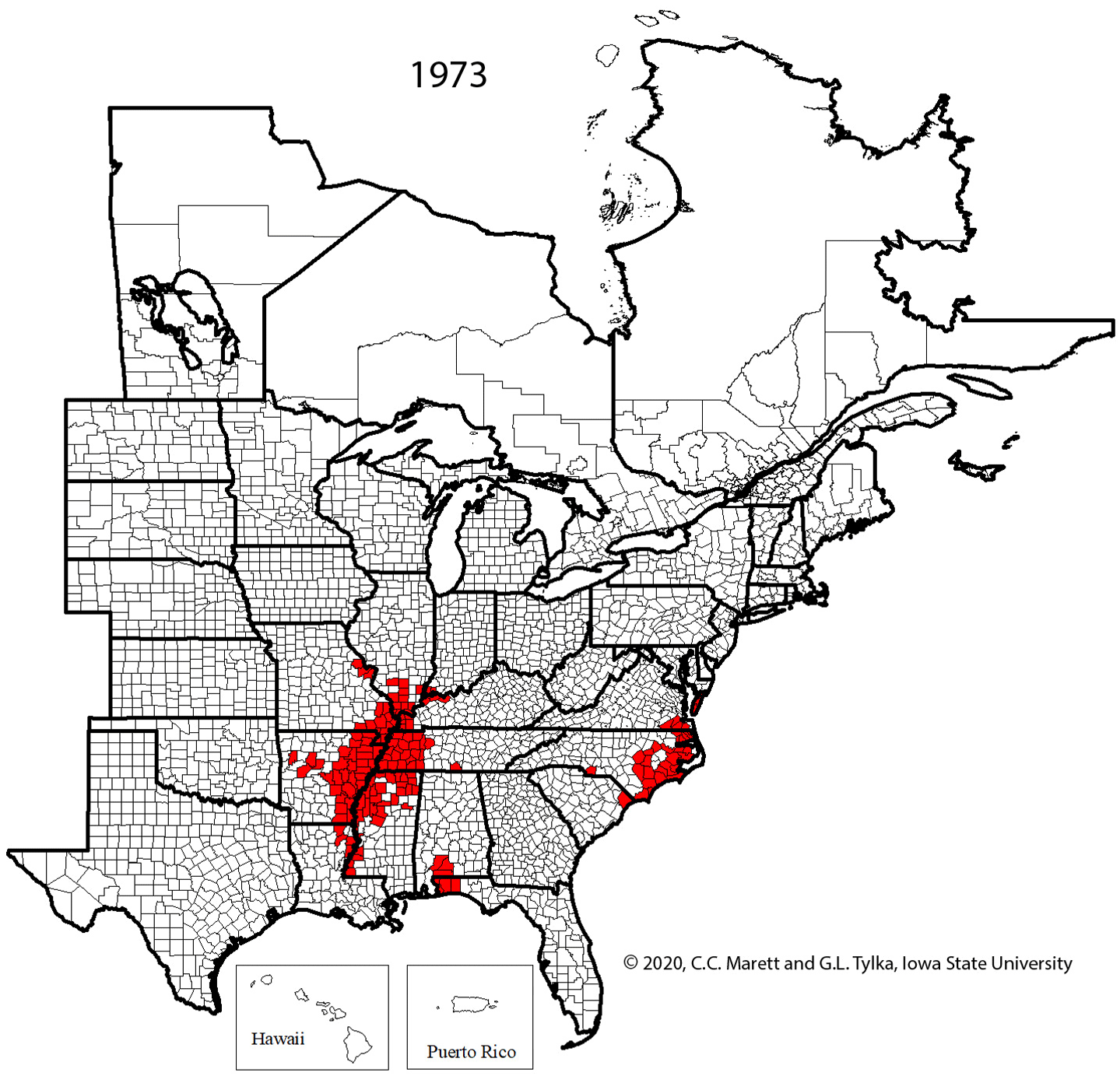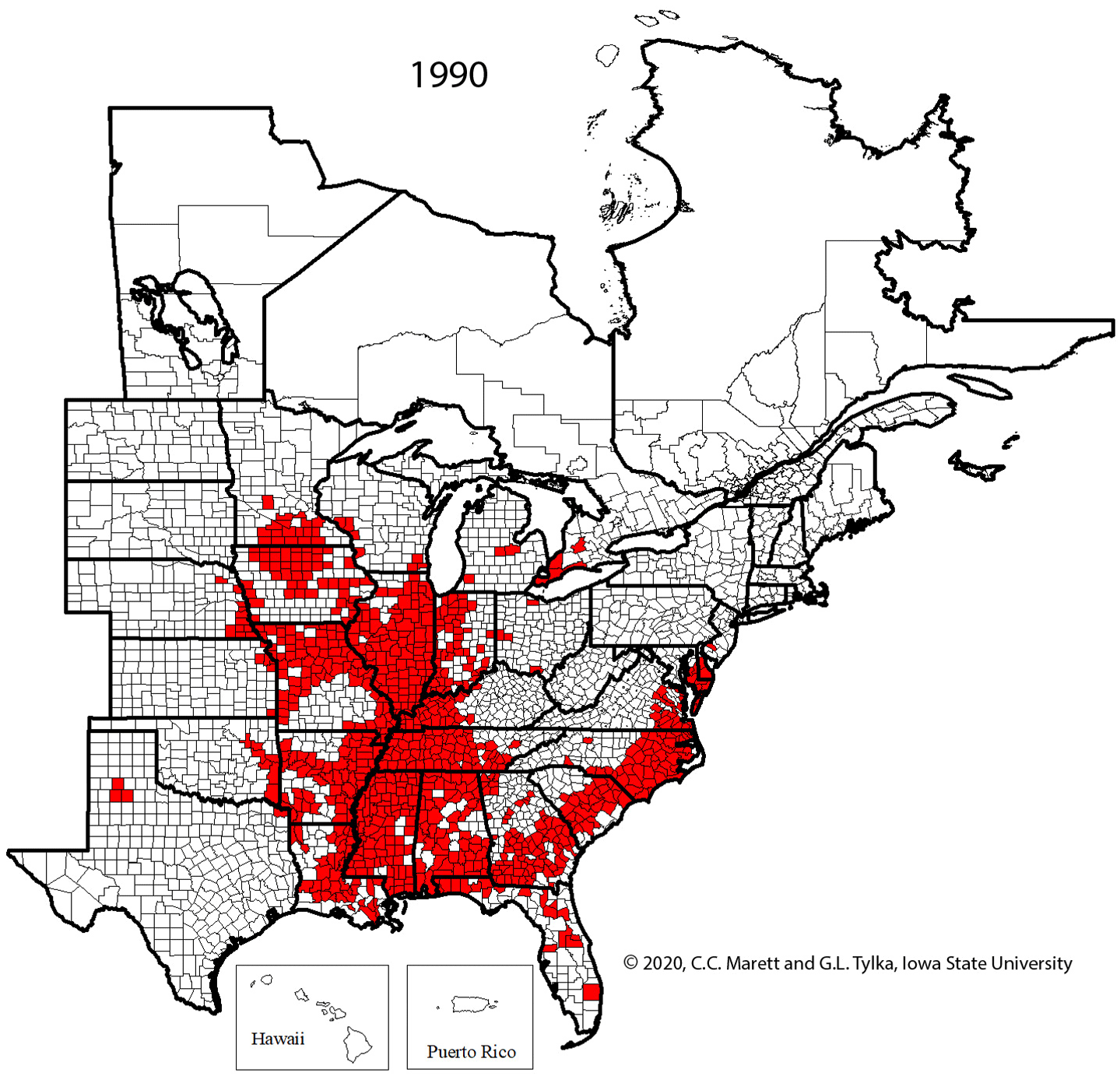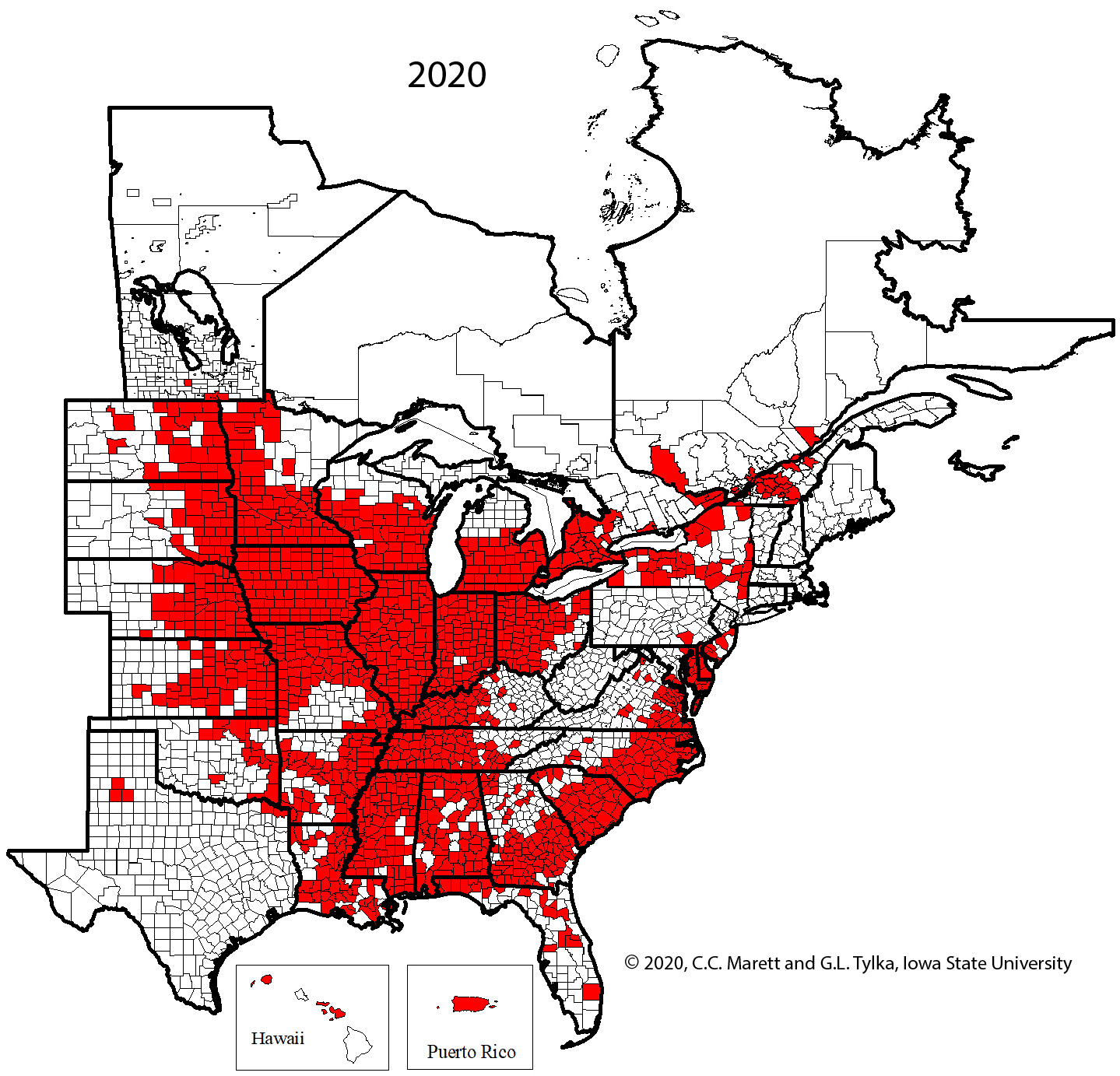
Supplemental Video 1. Corresponding animation for Figure 2 showing SCN spread since 1954.