Full text loading...
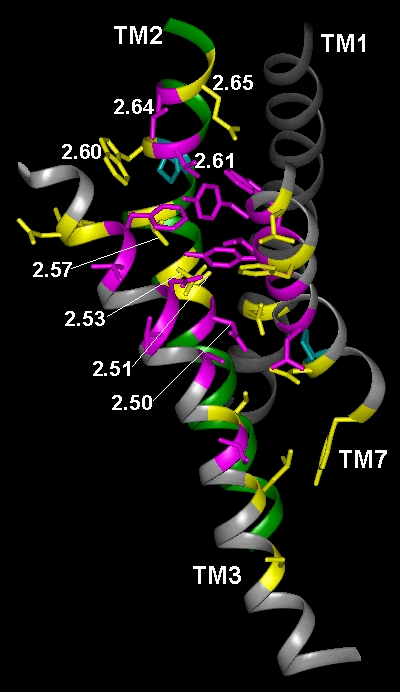
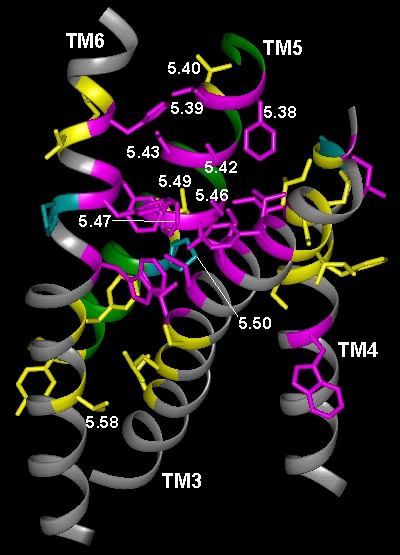
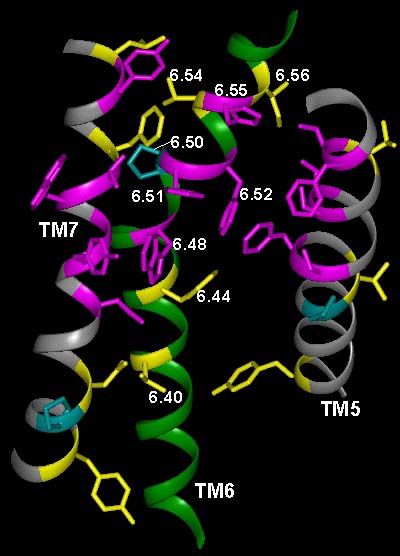
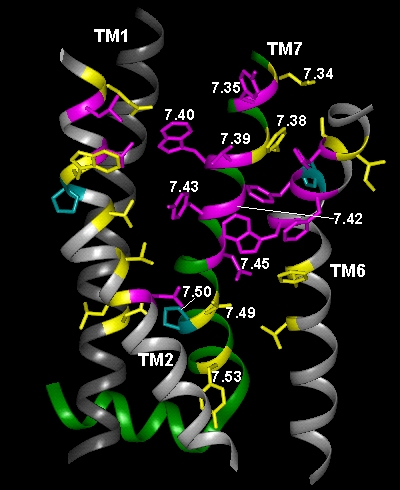
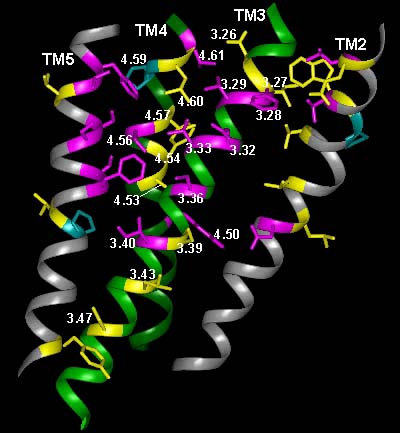
In the current chapter, we review approaches to the identification of the residues forming the binding sites for agonists, antagonists, and allosteric modulators in the family of aminergic G protein–coupled receptors (GPCRs). We then review the structural bases for ligand binding and pharmacological specificity based on the application of these methods to muscarinic cholinergic, adrenergic, dopaminergic, serotonergic, and histaminergic receptors, using the high resolution rhodopsin structure as a template. Furthermore, we propose a critical role of the second extracellular loop in forming the binding site for small molecular weight aminergic ligands, much as this loop dives down into the binding-site crevice and contacts retinal in rhodopsin.

Article metrics loading...

Full text loading...


Data & Media loading...
Supplemental Material
Figure TM2 Figure TM5 Figure TM6 Figure TM7 Figure TM34 Download 3D molecular model (VRML Format).