Full text loading...
Amphibians and reptiles as a group are often secretive, reach their greatest diversity often in remote tropical regions, and contain some of the most endangered groups of organisms on earth. Particularly in the past decade, genetics and genomics have been instrumental in the conservation biology of these cryptic vertebrates, enabling work ranging from the identification of populations subject to trade and exploitation, to the identification of cryptic lineages harboring critical genetic variation, to the analysis of genes controlling key life history traits. In this review, we highlight some of the most important ways that genetic analyses have brought new insights to the conservation of amphibians and reptiles. Although genomics has only recently emerged as part of this conservation tool kit, several large-scale data sources, including full genomes, expressed sequence tags, and transcriptomes, are providing new opportunities to identify key genes, quantify landscape effects, and manage captive breeding stocks of at-risk species.

Article metrics loading...

Full text loading...
Literature Cited


Data & Media loading...
Supplemental Material
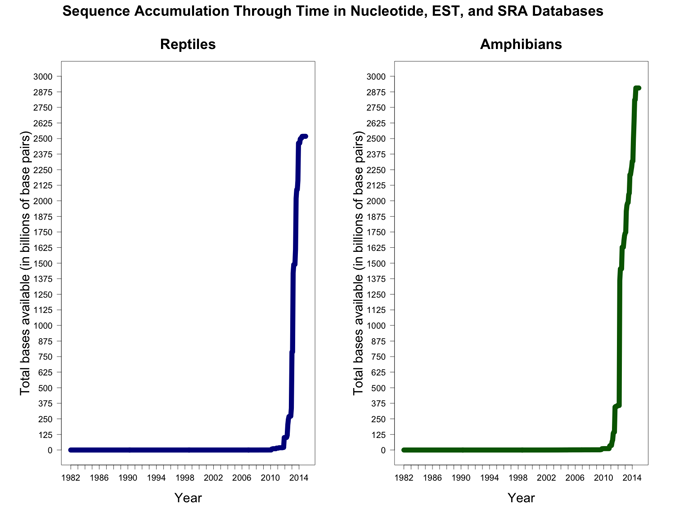
Supplemental Figure 1.